The development of the periodic table of elements was made possible by the pioneering work of renowned Russian chemist Dmitri Mendeleev. His path to this outstanding accomplishment, though, was not without difficulties. This article examines Mendeleev’s early years, going in-depth on his upbringing, education, and difficulties. Mendeleev’s life is one of tenacity and scientific brilliance, from his modest beginnings to the momentous discovery that revolutionized the field of chemistry. Join us as we examine the life of a man who influenced how we perceive the universe’s fundamental elements.
Early Life and Education of Dmitri Mendeleev
The developer of the periodic table of elements, Dmitri Mendeleev, was born in Tobolsk, Siberia, on February 8, 1834. His mother was from a family of merchants, and his father was a teacher. He came from a modest, mixed-race family. Mendeleev’s parents, despite their modest means, placed a high value on education and instilled a lifelong love of learning in him.
Mendeleev demonstrated a remarkable aptitude for mathematics and the sciences even as a young child. He went to the Gymnasium in Tobolsk, where his academic prowess really started to show. Tragically, his father’s blindness brought about financial hardship for the family. Despite these challenges, Mendeleev managed to secure a scholarship to continue his education at the prestigious University of Moscow, where he hoped to further his studies in chemistry.
Rejection from the University of Moscow: A Setback for Mendeleev
Mendeleev submitted an application to the University of Moscow in 1850, eager to pursue his love of chemistry and leave his mark on the scientific world. He hoped that his outstanding grades and intense interest in the subject would help him get accepted to the university.
Mendeleev received a rejection letter from the University of Moscow, which sadly crushed his dreams. Although the precise causes of his rejection are unknown, it is thought that his unconventional thinking and self-directed learning style clashed with the established academic system of the day.
Mendeleev refused to let the rejection determine his future, despite the fact that it was undoubtedly discouraging. He chose a different route in his quest for knowledge because he was determined to keep learning. Mendeleev immersed himself in self-directed studies, tirelessly exploring various scientific disciplines and refining his understanding of chemistry.
Journey of Mendeleev: Looking into Chemistry and Developing His Ideas
Mendeleev didn’t get the formal education he had hoped for, but his enthusiasm for chemistry was stronger than ever. In his improvised home laboratory, he started running experiments as he devoted his life to learning the secrets of the elements.
Mendeleev began to form his own theories and hypotheses about the nature of chemical elements through his independent research. He questioned the validity of the current categorization schemes and searched for a more thorough and systematic method of classifying the elements according to their properties.
Mendeleev collaborated with other scientists and participated in scholarly debates because of his insatiable desire for knowledge. He engaged in discussions with influential members of the scientific community in an effort to constantly improve and test his own theories. Mendeleev’s ground-breaking work was greatly influenced by his willingness to work with others and his capacity for taking in new information.
The Periodic Table: A Breakthrough in Understanding the Elements
Mendeleev was acutely aware of the disorderly state of the existing knowledge about the elements as he dove deeper into his studies. He understood the urgent need for an organized framework that would arrange these basic components of matter and help scientists better understand their characteristics and interactions.
Mendeleev came up with a plan to arrange the elements according to their atomic weights and recurring patterns in properties, drawing on his vast knowledge and keen insights. As a result of his discovery that some characteristics recurred periodically, the periodic table of elements as we know it today was created.
Mendeleev’s periodic table was extremely predictive, which was one of its most amazing features. Mendeleev boldly predicted the existence and characteristics of elements that were later discovered and confirmed by other scientists by leaving gaps in his table for yet-to-be-discovered elements. This foresight demonstrated his brilliance as a scientific thinker while also supporting the validity of his periodic table.
The progression of Dmitri Mendeleev from being rejected to creating the periodic table is evidence of his unwavering resolve, independent thinking, and ingenuity. His contributions transformed the study of chemistry and gave researchers a potent tool to decipher the mysteries of the elements. And even though Mendeleev was not admitted to the Moscow University, his contributions to science have had a lasting impact.
Key Features of Mendeleev’s Periodic Table
Mendeleev was brilliant because he could see patterns where others saw chaos. He arranged the elements into rows and columns according to their atomic weights, making comparison and analysis simple. This arrangement set the way for the current periodic table by helping scientists in making sense of the vast array of elements that were known at the time.
Mendeleev’s arrangement of elements with comparable properties in one group was one of its remarkable aspects. This not only improved the appearance of the table but also revealed relationships between elements that had previously been hidden. Suddenly, it became clear that elements in the same group shared similar chemical behaviors and properties, making it easier for chemists to make predictions and understand the behavior of new and undiscovered elements.
Initial Reactions and Scientific Acceptance of the Periodic Table
Mendeleev’s periodic table was initially met with doubt and even mockery by Scientists. Some scientists dismissed it as little more than an intriguing theory with no real world application. Mendeleev, however, didn’t let that discourage him. His table was continually improved, he gathered more data to back up his claims, and he gradually won over his detractors.
There are always going to be disagreements with groundbreaking scientific theories. Some scientists disagreed with Mendeleev’s choice to group elements solely by atomic weight, contending that additional considerations should have been made. There were also a lot of arguments about where to put certain components and where to draw the lines between various groups. But despite their ferocity, these discussions ultimately served a useful purpose and improved and strengthened the periodic table over time.
Support for Mendeleev’s work increased as more proof mounted and experts realized how useful his periodic table was in real-world applications. Scientists quickly realized the strength and beauty of his system. Worldwide, chemistry classrooms and laboratories quickly adopted the periodic table, and Mendeleev’s name came to represent scientific brilliance.

story of the youngest mother in the world at age of five - Lina Medina
Lina Medina, a five-year-old Peruvian girl, became the youngest mother in history in 1939 when she gave birth to a boy.

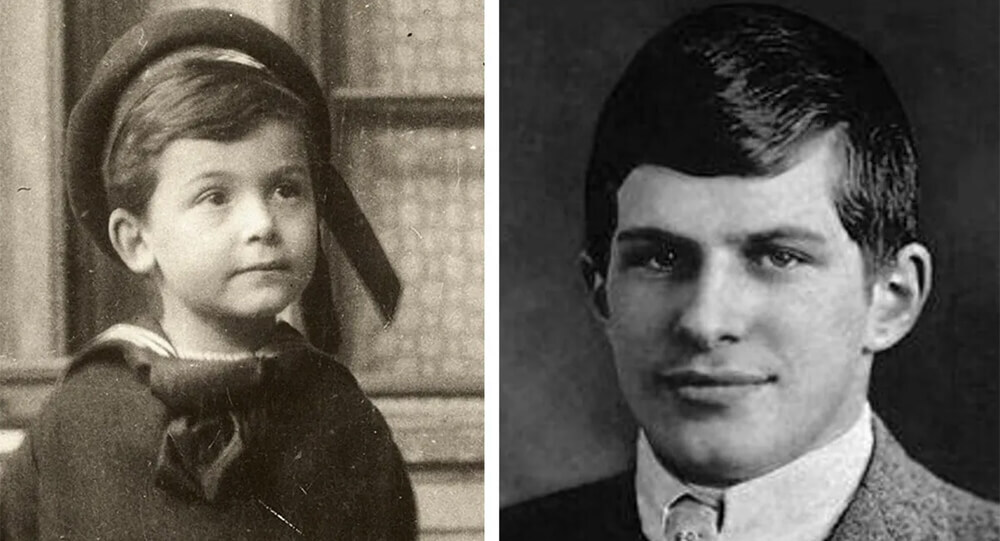
William James Sidis: The smartest person yet forgotten by people
William James Sidis, who was only 11 years old when he enrolled in Hardvard, finished his primary and secondary schooling in less than a year. He knew eight foreign languages by the age of eight and even invented his own language, "vedergood."

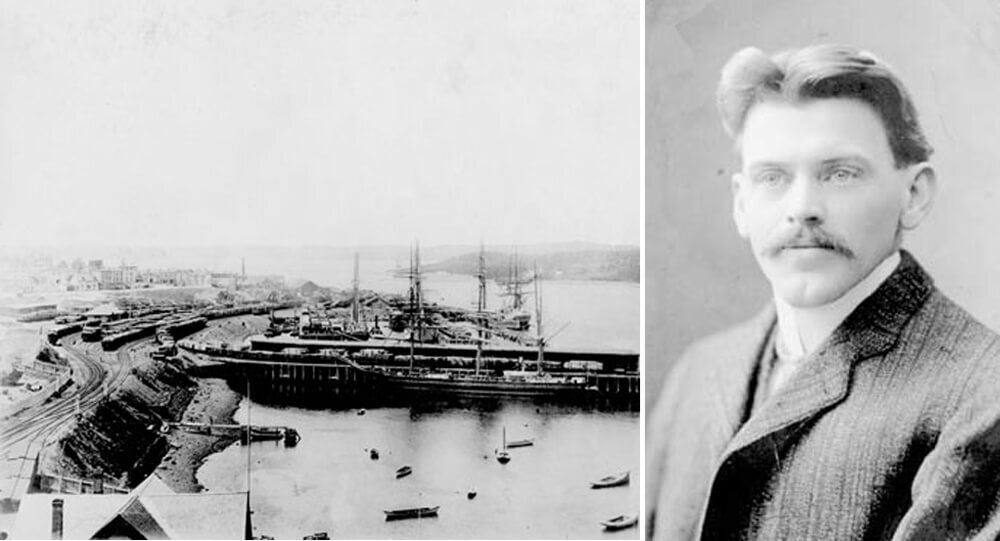
Vince Coleman, a railway dispatcher, sacrificed his own life
Vince Coleman, a railway dispatcher, sacrificed his life in order to warn an incoming train of an imminent explosion. His telegraph said “Hold up the train. Ammunition ship afire in harbor making for Pier 6 and will explode. Guess this will be my last message. Good-bye, boys.” He saved 300 lives.

Sylvan Goldman: The Visionary Who Revolutionized Shopping with the Cart
The inventor of shopping carts, Sylvan Goldman, had to hire several male and female models to push carts around in his store, demonstrate their utility, and explain their use to other customers, due to not catching on initially.

Underground Railroad to Mexico freed thousands of slaves in 1829
Slavery was abolished in Mexico in 1829. Slaves were escaping to Mexico, and slaveholders in the US were aware of this. The US attempted to get Mexico to sign a fugitive slave treaty, which would have required Mexico to send back escaped slaves to the US. But, Mexico refused, arguing that slaves were free as soon as they set foot on Mexican soil.

What Was the Beast of Gévaudan?
Between 1764 and 1767, a mysterious animal called the Beast of Gévaudan terrorized the French village called Gévaudan. It attacked and killed about 100 adults and children. While most believe it was a wolf, some say it may have been a wolf-dog hybrid, hyena or even a lion, but without any genetic evidence, the beast will remain a mystery forever.

The true story of Josephine Myrtle Corbin, the lady born with four legs and two private parts
Josephine Myrtle Corbin, an American sideshow performer born in 1868, had a rare condition known as dipygus, which caused her to have four legs, each smaller inner leg paired with one of her outer legs. Corbin joined the sideshow circuit, captivating audiences as the "Four-Legged Girl from Texas."

A Brief History of the PlayStation Gaming Console
Sony's PlayStation was never meant to be an actual product. Instead, it was intended to be a CD-ROM console that would support Nintendo games. However, when Nintendo backed out of the deal at the last minute, Sony went ahead and launched what soon became one of the most successful gaming consoles of all time.

Henry Ford, The man popularizing the concept of the weekend off
Henry Ford was the first Industrial Giant to give his employees both Saturday and Sunday off in the hope of encouraging more leisurely use of automobiles and thus popularizing the concept of the "weekend."

Saudi Arabia camel carvings dated to prehistoric era
Archaeologists were shocked to discover that a series of camels carved into desert rock faces in north-western Saudi Arabia are actually prehistoric, dating from 7,000-8,000 years ago - before either the Pyramids of Giza or Stonehenge were built.

Charlie Brown and Franz Stigler incident: Enemy became friends
During WWII, a German pilot spotted an American pilot’s crippled plane in the sky. Tailing it, he noticed that gunner was dead, crew injured, and they posed no threat. Instead of destroying the plane, he led it to safety. 40 years later, the two pilots reunited.

How European Rabbits Took over Australia
In 1859, wealthy settler Thomas Austin released 13 wild rabbits on his Australian estate. By 1920, their population grew to 10 billion.

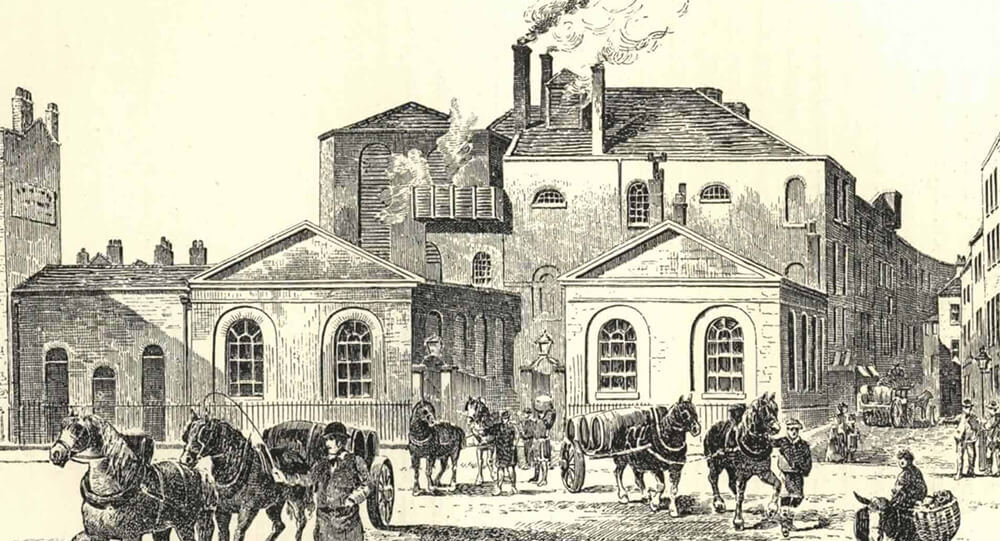
The 1814 London beer flood
In 1814, there was a beer flood in London when a tank containing more than 300,000 gallons ruptured in which 8 people drowned.

Remembering the 1945 Empire State Building Disaster: When a Plane Met Skyscraper
An airplane crashed into the Empire State Building in 1945. Among other damage, plane parts severed the cables of an elevator and the woman inside fell over 70 stories. She lived and holds the world record for the longest survived elevator fall.

Inside The Mysterious Death Of The Famed Gothic Writer Edgar Allan Poe
Hours before his death Edgar Allen Poe was found on the streets of Baltimore. He was incoherent, wearing another man’s clothes, and unable to explain how he got there. The cause of his death is an unsolved mystery.

Max Headroom Incident: America’s Creepiest TV Hack
In 1987 a man hijacked a television station during an episode of Dr. Who and wore a Max Headroom mask and uttered nonsense, and he still hasn’t been caught

Poto And Cabengo: The Secret Language Of Twins
Poto and Cabengo, as the two girls called each other, communicated in their own language. The twins were ignored by their parents and secluded from the outside world because their father felt they were developmentally retarded, and their unique language evolved as a result of that neglect.

Terry Fox, a 21-year-old one-legged cancer patient who ran 143 days before dying
Terry Fox was a 21-year-old one-legged cancer patient who ran 3,339 miles across Canada in 143 days before dying.

Why This Belgian Bar Makes You Trade Your Shoe for a Beer
To prevent tourists from stealing their beer glasses, some bars in Belgium require people to hand over one of their shoes as a deposit which is then put in a basket and hung from the ceiling. These shoe baskets have also become an attraction.

Shizo Kanakuri’s 1912 Olympic Marathon Finished 54 Years
At the 1912 Olympics, a marathon runner quit and went home to Japan without telling officials and was considered a missing person in Sweden for 50 years. In 1966, he was invited to complete the marathon. His time: 54 years, 8 months, 6 days, 5 hours, 32 minutes, and 20.379 seconds.

Knockers-up: waking up the Industrial Britain's Workers in 1900-1941
Before alarm clocks were invented, there was a profession called a knocker-up, which involved going from client to client and tapping on their windows (or banging on their doors) with long sticks until they were awake. It lasted into the 1920s.

Story of Kathrine Switzer: the first woman to run in Boston Marathon
Before women were allowed to run in the Boston Marathon, Kathrine Switzer participated. A race official attempted to forcefully remove her from the race in 1967, but her boyfriend pushed him down. She was the first female finisher who had a numbered entry in the race.

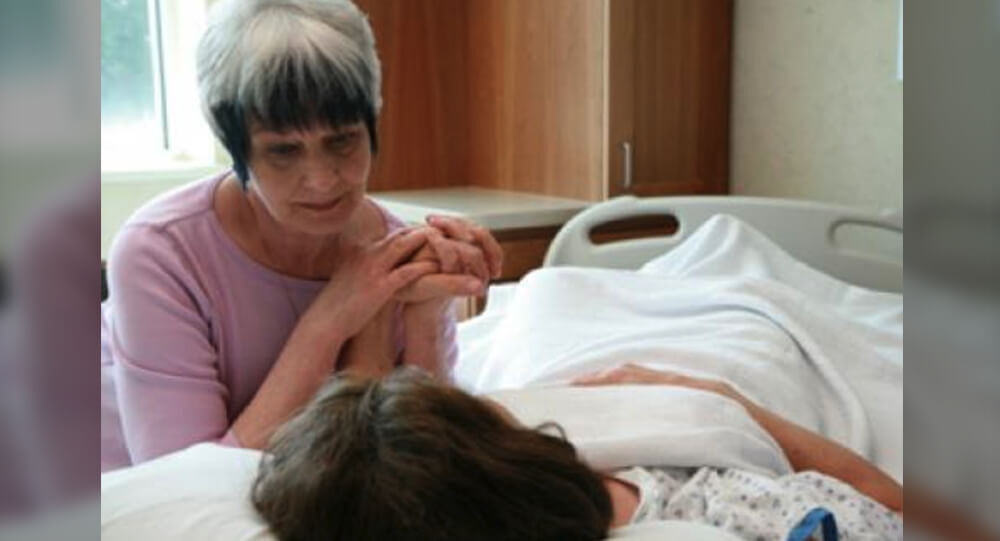
The History Behind the “No One Dies Alone” Program
In 1986, while doing a night shift at the hospital, Sandra Clarke, a registered nurse, was asked by an elderly patient to stay. She promised to be back after checking on her other patients, but by the time she returned, the gentleman had passed away. Clarke became one of the key figures in launching No One Dies Alone, a program that allows volunteers to sit with terminal patients who have no one else.

Nordlingen, The Town Inside A Meteorite Crater With Millions Of Meteorite Diamonds
The German town of Nördlingen is embedded with 72,000 tons of microscopic diamonds. About 15 million years ago, a meteorite hit this region, and the impact created a massive depression and formed rocks containing diamonds, glass, and crystals. The town was built in the impact crater sometime around 898 CE.

Moondyne Joe: The story of Australia's most notorious prison escapee
A man named Joseph Bolitho Johns (A.K.A Moondyne Joe) broke out of Australian prisons so many times that the police were compelled to build a special cell just for him. He escaped from that as well.