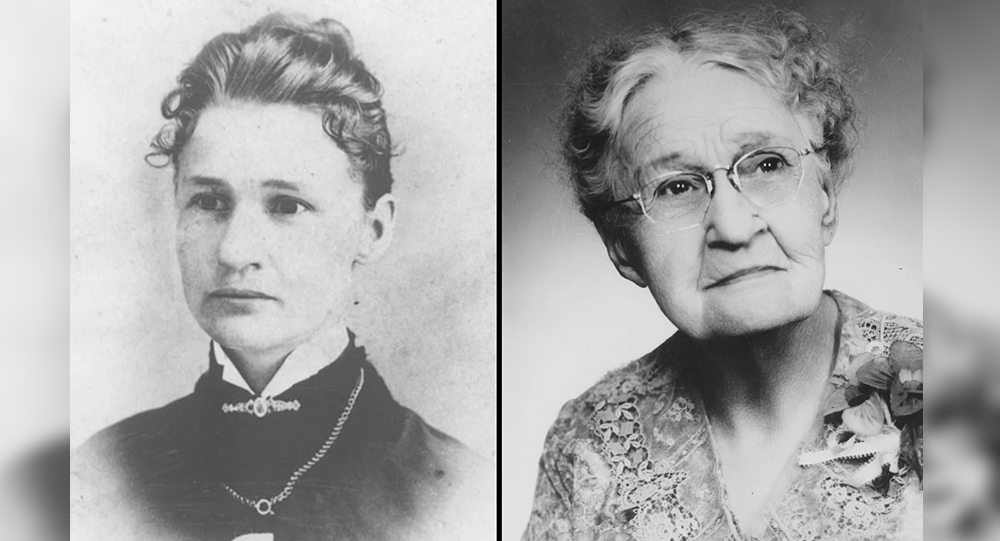The development of the periodic table of elements was made possible by the pioneering work of renowned Russian chemist Dmitri Mendeleev. His path to this outstanding accomplishment, though, was not without difficulties. This article examines Mendeleev’s early years, going in-depth on his upbringing, education, and difficulties. Mendeleev’s life is one of tenacity and scientific brilliance, from his modest beginnings to the momentous discovery that revolutionized the field of chemistry. Join us as we examine the life of a man who influenced how we perceive the universe’s fundamental elements.
Early Life and Education of Dmitri Mendeleev
The developer of the periodic table of elements, Dmitri Mendeleev, was born in Tobolsk, Siberia, on February 8, 1834. His mother was from a family of merchants, and his father was a teacher. He came from a modest, mixed-race family. Mendeleev’s parents, despite their modest means, placed a high value on education and instilled a lifelong love of learning in him.
Mendeleev demonstrated a remarkable aptitude for mathematics and the sciences even as a young child. He went to the Gymnasium in Tobolsk, where his academic prowess really started to show. Tragically, his father’s blindness brought about financial hardship for the family. Despite these challenges, Mendeleev managed to secure a scholarship to continue his education at the prestigious University of Moscow, where he hoped to further his studies in chemistry.
Rejection from the University of Moscow: A Setback for Mendeleev
Mendeleev submitted an application to the University of Moscow in 1850, eager to pursue his love of chemistry and leave his mark on the scientific world. He hoped that his outstanding grades and intense interest in the subject would help him get accepted to the university.
Mendeleev received a rejection letter from the University of Moscow, which sadly crushed his dreams. Although the precise causes of his rejection are unknown, it is thought that his unconventional thinking and self-directed learning style clashed with the established academic system of the day.
Mendeleev refused to let the rejection determine his future, despite the fact that it was undoubtedly discouraging. He chose a different route in his quest for knowledge because he was determined to keep learning. Mendeleev immersed himself in self-directed studies, tirelessly exploring various scientific disciplines and refining his understanding of chemistry.
Journey of Mendeleev: Looking into Chemistry and Developing His Ideas
Mendeleev didn’t get the formal education he had hoped for, but his enthusiasm for chemistry was stronger than ever. In his improvised home laboratory, he started running experiments as he devoted his life to learning the secrets of the elements.
Mendeleev began to form his own theories and hypotheses about the nature of chemical elements through his independent research. He questioned the validity of the current categorization schemes and searched for a more thorough and systematic method of classifying the elements according to their properties.
Mendeleev collaborated with other scientists and participated in scholarly debates because of his insatiable desire for knowledge. He engaged in discussions with influential members of the scientific community in an effort to constantly improve and test his own theories. Mendeleev’s ground-breaking work was greatly influenced by his willingness to work with others and his capacity for taking in new information.
The Periodic Table: A Breakthrough in Understanding the Elements
Mendeleev was acutely aware of the disorderly state of the existing knowledge about the elements as he dove deeper into his studies. He understood the urgent need for an organized framework that would arrange these basic components of matter and help scientists better understand their characteristics and interactions.
Mendeleev came up with a plan to arrange the elements according to their atomic weights and recurring patterns in properties, drawing on his vast knowledge and keen insights. As a result of his discovery that some characteristics recurred periodically, the periodic table of elements as we know it today was created.
Mendeleev’s periodic table was extremely predictive, which was one of its most amazing features. Mendeleev boldly predicted the existence and characteristics of elements that were later discovered and confirmed by other scientists by leaving gaps in his table for yet-to-be-discovered elements. This foresight demonstrated his brilliance as a scientific thinker while also supporting the validity of his periodic table.
The progression of Dmitri Mendeleev from being rejected to creating the periodic table is evidence of his unwavering resolve, independent thinking, and ingenuity. His contributions transformed the study of chemistry and gave researchers a potent tool to decipher the mysteries of the elements. And even though Mendeleev was not admitted to the Moscow University, his contributions to science have had a lasting impact.
Key Features of Mendeleev’s Periodic Table
Mendeleev was brilliant because he could see patterns where others saw chaos. He arranged the elements into rows and columns according to their atomic weights, making comparison and analysis simple. This arrangement set the way for the current periodic table by helping scientists in making sense of the vast array of elements that were known at the time.
Mendeleev’s arrangement of elements with comparable properties in one group was one of its remarkable aspects. This not only improved the appearance of the table but also revealed relationships between elements that had previously been hidden. Suddenly, it became clear that elements in the same group shared similar chemical behaviors and properties, making it easier for chemists to make predictions and understand the behavior of new and undiscovered elements.
Initial Reactions and Scientific Acceptance of the Periodic Table
Mendeleev’s periodic table was initially met with doubt and even mockery by Scientists. Some scientists dismissed it as little more than an intriguing theory with no real world application. Mendeleev, however, didn’t let that discourage him. His table was continually improved, he gathered more data to back up his claims, and he gradually won over his detractors.
There are always going to be disagreements with groundbreaking scientific theories. Some scientists disagreed with Mendeleev’s choice to group elements solely by atomic weight, contending that additional considerations should have been made. There were also a lot of arguments about where to put certain components and where to draw the lines between various groups. But despite their ferocity, these discussions ultimately served a useful purpose and improved and strengthened the periodic table over time.
Support for Mendeleev’s work increased as more proof mounted and experts realized how useful his periodic table was in real-world applications. Scientists quickly realized the strength and beauty of his system. Worldwide, chemistry classrooms and laboratories quickly adopted the periodic table, and Mendeleev’s name came to represent scientific brilliance.

Ea-Nasir: world's oldest written customer complaint
This clay tablet, written in cuneiform, is the oldest known written customer complaint about the delivery of poor quality copper ingots. Originally from ancient Babylon, the tablet dates back to 1750 BCE, and it was written by a customer named Nanni to a merchant named Ea-Nasir. It is currently housed in the British Museum.

Medieval Medicine: A 1,000-year-old onion and garlic salve kills modern bacterial superbugs
Scientists recreated an Anglo-Saxon manuscript-based 9th century onion and garlic eye remedy and discovered that it killed 90% of antibiotic-resistant staph bacteria (MRSA).

The Baltic Way: the longest unbroken human chain in history
On August 23, 1989, about 2 million people from Latvia, Estonia, and Lithuania formed a human chain that united all 3 countries to show the world their desire to escape the Soviet Union and the communism that brought only suffering and poverty. This power stretched 600 km.

How Sleep Deprivation Was Once Used as Torture
Sleep deprivation, long before modern interrogation techniques, was considered a “clean” and effective form of torture—leaving no physical scars, yet breaking minds with haunting silence. Victims endured days and nights without rest, leading to vivid hallucinations, disorientation, and psychological torment. This article traces the dark history of sleep deprivation as a weapon, examines the science behind its effects on the brain, and shines a light on the painful balance between human endurance and cruelty in the annals of coercion.

The Assassination Of King Alexander
The assassination of King Alexander of Yugoslavia marked a pivotal moment in the country's history. This article delves into the rise and reign of King Alexander, exploring his early life and ascension to the throne. It also examines the political and social climate in interwar Yugoslavia, setting the stage for the tensions and challenges that ultimately culminated in his tragic assassination. By understanding the context in which this event unfolded, we can better grasp the significance and impact it had on the nation and its future.

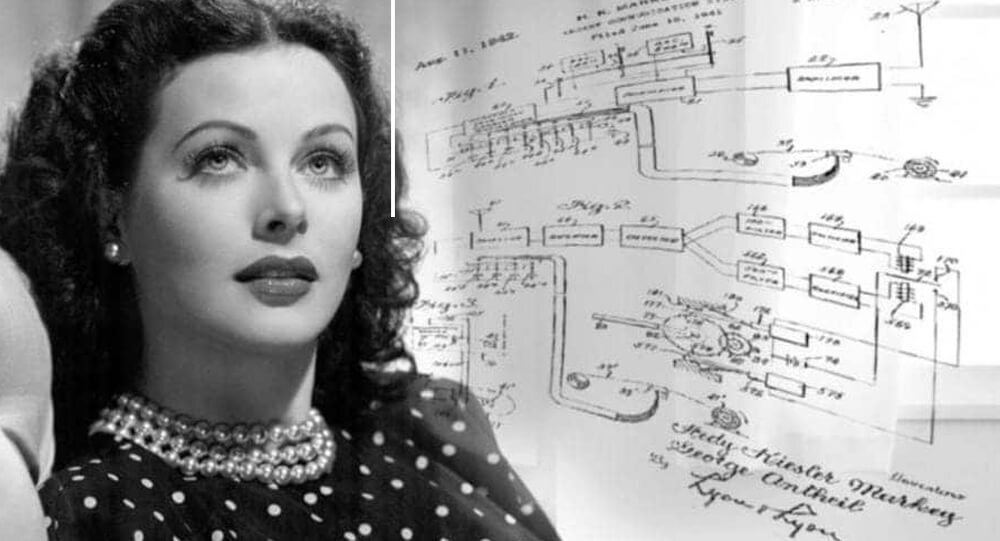
Hedy Lamarr, A Hollywood actress who also a mathematician and inventor
Hollywood actress Hedy Lamarr was also a mathematician and the inventor of frequency hopping spread spectrum, a technology still used for bluetooth and wifi

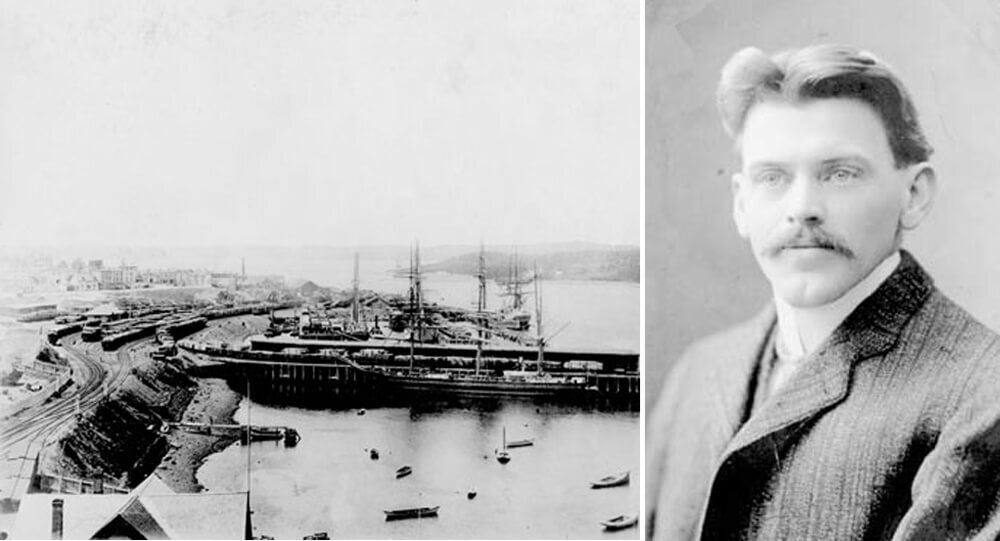
Robert Odlum, the first person to jump off the Brooklyn Bridge
The first person to jump off the Brooklyn Bridge was a professional high diver who "wanted to demonstrate that people did not die simply by falling through the air, thus encouraging people to be willing to jump from a burning building into a net." He proved himself correct by safely falling 135 feet through the air and dying only when he hit the water.

Poto And Cabengo: The Secret Language Of Twins
Poto and Cabengo, as the two girls called each other, communicated in their own language. The twins were ignored by their parents and secluded from the outside world because their father felt they were developmentally retarded, and their unique language evolved as a result of that neglect.

The day Iceland's women went on strike
Icelandic women went on strike for equal rights on October 24, 1975. 90% of women walked out of their jobs and homes, effectively shutting down the entire country. The men were struggling to keep up. The following year, Parliament passed a law requiring equal pay. Iceland elected the world's first female President five years later. Iceland now has the highest gender equality rate in the world.

Martin Couney, Saved Thousands of Premature Babies Wasn’t a Doctor at All
Martin Couney never qualified as a medical doctor. However, in the 1900s, he saved thousands of premature babies by exhibiting them in incubators at his Coney Island sideshow. Over the course of his career, he is said to have saved about 6,500 babies that had previously been written off by mainstream medicine.

Susanna Salter: The Trailblazing Story of America’s First Female Mayor
In 1887, Susanna Salter became the first female mayor in the United States, elected in Argonia, Kansas. Her nomination was initially a prank by men opposing women in politics. However, she won by a landslide and served effectively, inspiring the women’s suffrage movement and breaking barriers for women in leadership.

The Bizarre (And Magical) Duel Between Chung Ling Soo And Ching Ling Foo
Ching Ling Foo and Chung Ling Soo were two magicians from the early 20th century who were bitter rivals. While Ching Ling Foo was genuinely Chinese, Chung Ling Soo was actually a New Yorker named William Robinson.

Juliane Koepcke: The Teenager Who Fell 10,000 Feet And Trekked The Jungle to survive
In 1971, a high school student was sucked out of an airplane after it was struck by lightning. She fell 10,000 feet to the ground while still strapped to her chair and survived. Only to endure a 9-day trek to the nearest civilization.

Atomic Tourism: In the 1950s, nuclear tests in Las Vegas served as a draw for tourists
Between 1950 and 1960, Las Vegas offered “Atomic Tourism” in which guests could watch atomic bombs being tested in the desert as a form of entertainment.

What exactly was the US's 'Ghost Army' during WWII?
During WW2, there was a special unit of men dubbed the ‘Ghost Army’. The unit was made of artists, creative and engineers and their job was to create deception about the enemy. From inflatable tanks to phony convoys to scripted conversations in bars intended to spread disinformation, they used all possible tricks to fool the enemy.

Inside The Mysterious Death Of The Famed Gothic Writer Edgar Allan Poe
Hours before his death Edgar Allen Poe was found on the streets of Baltimore. He was incoherent, wearing another man’s clothes, and unable to explain how he got there. The cause of his death is an unsolved mystery.

What Was the Beast of Gévaudan?
Between 1764 and 1767, a mysterious animal called the Beast of Gévaudan terrorized the French village called Gévaudan. It attacked and killed about 100 adults and children. While most believe it was a wolf, some say it may have been a wolf-dog hybrid, hyena or even a lion, but without any genetic evidence, the beast will remain a mystery forever.

Charlie Brown and Franz Stigler incident: Enemy became friends
During WWII, a German pilot spotted an American pilot’s crippled plane in the sky. Tailing it, he noticed that gunner was dead, crew injured, and they posed no threat. Instead of destroying the plane, he led it to safety. 40 years later, the two pilots reunited.

3 men lived on top of a billboard in tents for almost 9 months
From 1982-1983, three men in Allentown PA competed in a radio contest in which they lived on top of a billboard in tents. Whoever stayed up longest would win a house. Due to economic pressure from the recession, none of the contestants wanted to give up, so the contest lasted almost 9 months.

Archaeologists Uncover 2,000-Year-Old Amazonian Cities Using Lidar Technology
Deep in the Ecuadorian Amazon, archaeologists have uncovered an ancient network of urban settlements once inhabited by the Upano people about 2,000 years ago. Using cutting-edge lidar technology, these discoveries reveal a highly organized society featuring sophisticated agricultural systems, drainage canals, and extensive road networks. This transformative find challenges long-held assumptions about ancient Amazonian societies and sheds light on a complex civilization thriving in one of the world’s most biodiverse regions.

Vince Coleman, a railway dispatcher, sacrificed his own life
Vince Coleman, a railway dispatcher, sacrificed his life in order to warn an incoming train of an imminent explosion. His telegraph said “Hold up the train. Ammunition ship afire in harbor making for Pier 6 and will explode. Guess this will be my last message. Good-bye, boys.” He saved 300 lives.

The World’s First Seismograph: How Ancient China Detected Earthquakes 1,800 Years Ago
Over 1,800 years ago, long before modern technology, the ancient Chinese astronomer and inventor Zhang Heng created the world’s first seismograph in 132 AD. This ingenious bronze device could detect distant earthquakes by releasing small balls from dragons’ mouths into toads’ mouths—each indicating a different compass direction. Its historic detection of an earthquake 400 miles away astonished the imperial court and transformed the way societies understood and responded to seismic events.

Quaker Oats Fed Children with Radioactive Oatmeal
In the 1940s and 1950s, Quaker Oats and MIT conducted experiments on radioactive iron and calcium-containing cereal. The diet was part of a study to see if the nutrients in Quaker oatmeal traveled throughout the body. In January 1998, a $1.85 million settlement was reached for 30 victims who came forward.

Tunnels Dug by ancient giant sloths, A South American Megafauna
For years, scientists didn’t know what caused mysterious cave networks in South America. In 2010, they learned that the caves were actually tunnels dug by ancient giant sloths

How 18th Century Women’s Rights Movements Shaped Modern Equality
The 18th century marked a turning point in the quest for women’s rights, as passionate voices challenged centuries of gender inequality and laid the groundwork for modern feminism. From pioneers like Mary Wollstonecraft to revolutionary declarations and early advocacy, this era sparked debates on education, political participation, and social justice that continue to resonate today. Journey through the origins of women’s rights movements and discover how their bold ideas shaped the fight for equality.